Sulfuric acid
In this article, we explain the basics of sulfuric acid-based anodizing baths and provide tips on how to determine and adjust the concentration.
Contents
- 1. Basics of choosing the acid concentration in the anodizing bath
- 2. Measuring the acid concentration
- 3. Calculation of dilution
- 4. Disposal of used anodizing baths
- 5. Table of sulfuric acid concentrations
1. Basics of choosing the acid concentration in the anodizing bath
The acid concentration – along with other parameters – has a significant influence on layer formation. As explained below, two competing processes generally occur in the bath, and by carefully choosing the concentration, one process can be favored over the other.
During layer formation, several processes occur simultaneously in the bath, of which only the two most important are relevant here:
- The layer growth with the formation of anodic ceramic by the reaction of electrolytically generated oxygen with the surface aluminum.
- The partial layer dissolution through the dissolution of parts of the formed layer by the existing sulfuric acid.
This leads to:
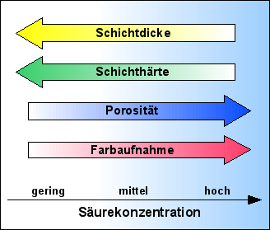
- The higher the acid concentration, the larger the pores in the layer, the slower the layer grows, and the softer it becomes.
- The lower the acid concentration, the smaller the pores, the faster the layer grows, and the harder it becomes.
- The smaller the pores and the fewer they are, the worse the dye absorption.
- The larger the pores and the more they are, the better the dye absorption.
The consequences are:
If a hard, thick layer is desired (hard anodizing), the acid concentration is set lower.
If the best possible dye absorption is desired – mainly for decorative purposes – a higher acid concentration is chosen.
Note:
By using our
„hard anodizing additive“, very good hard anodic layers can also be produced in baths with higher acid concentrations. More details can be found in our „TM-7 Hard Anodizing“
in the Download Area.
2. Measuring the acid concentration
2.1 Hydrometer / Sink Scale
The simplest, fastest, and most commonly used method is to determine the density of the acid bath using a hydrometer (sink scale). This uses the simple principle that a body sinks into a liquid until the weight of the displaced liquid equals the weight of the body itself. If the body is marked appropriately, the density can be read directly from these marks.
Usually, sealed glass hydrometers are used, which contain metal beads as weight and are calibrated accordingly. Since the density of liquids also depends on temperature, the values read on a hydrometer are always valid for a specific temperature, usually indicated on the scale.
Depending on the liquid to be measured, hydrometers are available for different density ranges: for light liquids like alcohol (density approx. 0.79 g/cm³, 790 g/L) dissolved in water, one uses a range from 0.79 (pure alcohol) to 1.00 (pure water = 1.00 g/cm³) to determine the concentration of pure alcohol in spirits. The closer the minimum and maximum values, the more precise the reading, but the narrower the range in which the hydrometer can be used. One should consider the possible highest or lowest densities and choose the hydrometer accordingly – or obtain several hydrometers with consecutive ranges to measure very precisely.
Measuring with a hydrometer is simple: the hydrometer is slowly lowered into the liquid to be measured, and the value at the liquid level is read. This density value is then converted to a percentage using a table.
2.2 Weight determination from volume
This is another simple method. A precise volume of the anodizing bath is measured, and its weight is determined. Using the simple formula
the density can be calculated, and from the table, the acid concentration can be determined.
Example:
50 cm³ of the anodizing bath is taken using a volumetric flask and weighed. With a result of 55.2 g, the density is calculated using the formula 55.2 g / 50 cm³ = 1.104 g/cm³. The table then gives an acid concentration of slightly over 15%.
Note that other substances such as dissolved aluminum or additives also affect the density, slightly distorting the result. However, due to the much higher sulfuric acid concentration, the deviations are usually negligible. For precise acid determination, a so-called titration must be carried out, as briefly described in the next section.
2.3 Titration
Titration determines the amount of an unknown acid by adding a base of known concentration until the solution reacts neutrally (pH 7). The pH is monitored either with special dye indicators, which change color at neutrality, or (more accurately today) with electronic pH meters.
Titration is much more accurate than the previously described density measurement methods, as only the actual acid is measured, not dissolved aluminum.
However, it is more labor-intensive and usually disproportionate if only refreshing an existing bath. Therefore, we omit a detailed procedure here. Numerous resources are easily found online for those interested.
3. Calculation of dilution
Since commercially available acid often has a different (=higher) concentration than needed, an important practical question is:
"How much water must I add to acid of concentration X% to obtain acid of concentration Y%?"
The following simple steps explain this and demonstrate it with an example.
When diluting liquids, the calculation is practically always done using masses (or – incorrectly – "weight") of the components. This is because mixing volumes can change (non-linearly), while masses remain constant: 1 liter water + 1 liter acid does not yield exactly 2 liters of diluted acid, but 1 kg acid + 1 kg water still weighs exactly 2 kg. This phenomenon is called volume contraction.
To obtain a desired concentration, proceed as follows:
- Find the corresponding amount of sulfuric acid (column 3, "g H2SO4/l") for the desired concentration in the table and multiply it by the required volume in liters.
- Find similarly the amount of sulfuric acid per liter in the available acid.
- Divide the required amount (from step 1) by the amount available per liter (from step 2) to find how many liters of starting acid are needed.
- Finally, add distilled water to reach the desired total volume.

All acids heat up significantly when diluted, so they should be added slowly to plenty of cold water, never the reverse! For the above procedure, this means the calculated amount of acid (step 3) is first added slowly to a suitable amount of cold water, then the remainder is added to reach the desired final volume.
Do not forget: wear protective gloves, safety goggles, and old clothing!
Example: We have 38% battery acid and want to make 10 liters of 15% acid from it.
- Step 1: Look up the required amount of sulfuric acid per liter for 15%: 165.30 g. For 10 liters, multiply by 10:
10 x 165.30 g = 1653.0 g
- Step 2: Find the amount of sulfuric acid per liter in our 38% starting acid: 488.50 g
- Step 3: Divide the required amount (1653.0 g) by the available amount (488.50 g/L) to get the needed volume of battery acid:
1653.0 g / (488.50 g/L) = 3.38 litersMeasure this volume in a container.
- Step 4: To make 10 liters of acid but only need 3.38 liters of acid, first pour e.g., 5 liters of water, then slowly stir in the measured acid (3.38 liters). This yields about 8 liters of acid bath, then carefully add water to reach 10 liters – done!
4. Disposal of used anodizing baths
Disposal instructions for used anodizing baths can be found in our „TM-5 Disposal of Chemicals“ in the Download Area.
5. Table of sulfuric acid concentrations
Below is a table of sulfuric acid concentrations. Under "Notes," the recommended ranges for each acid concentration are indicated.
| H2SO4 in % |
Density ρ (+20°C) in g/cm³ |
H2SO4 in g/l |
H2SO4 in mol/l |
Notes |
|---|---|---|---|---|
| 1 | 1,0051 | 10,05 | 0,103 | |
| 2 | 1,0118 | 20,24 | 0,206 | |
| 3 | 1,0184 | 30,55 | 0,312 | |
| 4 | 1,0250 | 41,00 | 0,418 | |
| 5 | 1,0317 | 51,59 | 0,526 | Hard Anodising (*1) |
| 6 | 1,0385 | 62,31 | 0,635 | |
| 7 | 1,0453 | 73,17 | 0,746 | |
| 8 | 1,0522 | 84,18 | 0,858 | |
| 9 | 1,0591 | 95,32 | 0,972 | |
| 10 | 1,0661 | 106,60 | 1,087 | |
| 11 | 1,0731 | 118,00 | 1,203 | |
| 12 | 1,0802 | 129,60 | 1,321 | Decorative Anodizing |
| 13 | 1,0874 | 141,40 | 1,442 | |
| 14 | 1,0947 | 153,30 | 1,563 | |
| 15 | 1,1020 | 165,30 | 1,685 | |
| 16 | 1,1094 | 177,50 | 1,810 | |
| 17 | 1,1168 | 189,90 | 1,936 | |
| 18 | 1,1234 | 202,40 | 2,063 | |
| 19 | 1,1318 | 215,00 | 2,192 | |
| 20 | 1,1394 | 227,90 | 2,324 | |
| 21 | 1,1471 | 240,90 | 2,456 | |
| 22 | 1,1548 | 254,10 | 2,591 | |
| 23 | 1,1626 | 267,40 | 2,726 | |
| 24 | 1,1704 | 280,90 | 2,864 | |
| 25 | 1,1783 | 294,60 | 3,004 | |
| 26 | 1,1862 | 308,40 | 3,144 | |
| 27 | 1,1942 | 322,40 | 3,287 | |
| 28 | 1,2023 | 336,60 | 3,432 | |
| 29 | 1,2104 | 351,00 | 3,579 | |
| 30 | 1,2185 | 365,60 | 3,728 | |
| 31 | 1,2267 | 380,30 | 3,878 | |
| 32 | 1,2349 | 395,20 | 4,029 | |
| 33 | 1,2432 | 410,30 | 4,183 | |
| 34 | 1,2515 | 425,50 | 4,338 | |
| 35 | 1,2599 | 441,00 | 4,496 | |
| 36 | 1,2684 | 456,60 | 4,656 | |
| 37 | 1,2769 | 472,50 | 4,818 | Battery acid |
| 38 | 1,2855 | 488,50 | 4,981 | |
| 39 | 1,2941 | 504,70 | 5,146 | |
| 40 | 1,3028 | 521,10 | 5,313 | |
| 41 | 1,3116 | 537,80 | 5,483 | |
| 42 | 1,3205 | 554,60 | 5,655 | |
| 43 | 1,3294 | 571,60 | 5,828 |
(*1) Using our hard anodizing additive, even baths for decorative (=colored) anodizing (12-20% acid) can be used for hard anodizing, so only one anodizing bath is needed.

